What Cell Has No Nucleus

| Cell biology | |
|---|---|
| Creature cell diagram | |
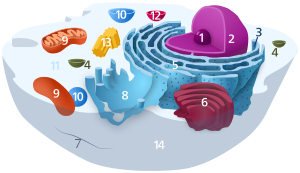 Components of a typical animal cell:
|
The cell nucleus (pl. nuclei; from Latin nucleus or nuculeus , meaning kernel or seed) is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, have no nuclei, and a few others including osteoclasts take many. The main structures making up the nucleus are the nuclear envelope, a double membrane that encloses the entire organelle and isolates its contents from the cellular cytoplasm; and the nuclear matrix (which includes the nuclear lamina), a network within the nucleus that adds mechanical support, much like the cytoskeleton supports the cell equally a whole.
The cell nucleus contains all of the prison cell'due south genome, except for the small amount of mitochondrial Dna and, in plant cells, plastid DNA. Nuclear DNA is organized as multiple long linear molecules in a complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these chromosomes are structured in such a way to promote cell function. The nucleus maintains the integrity of genes and controls the activities of the prison cell by regulating cistron expression—the nucleus is, therefore, the control eye of the cell.
Because the nuclear envelope is impermeable to large molecules, nuclear pores are required to regulate nuclear send of molecules beyond the envelope. The pores cross both nuclear membranes, providing a channel through which larger molecules must be actively transported by carrier proteins while assuasive gratis motion of small molecules and ions. Movement of large molecules such as proteins and RNA through the pores is required for both gene expression and the maintenance of chromosomes.
Although the interior of the nucleus does not contain any membrane-bound subcompartments, its contents are not uniform, and a number of nuclear bodies exist, made up of unique proteins, RNA molecules, and particular parts of the chromosomes. The best-known of these is the nucleolus, which is mainly involved in the assembly of ribosomes. Afterward being produced in the nucleolus, ribosomes are exported to the cytoplasm where they translate messenger RNA.
Structures
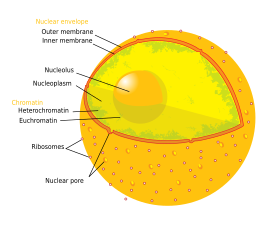
The nucleus contains nearly all of the jail cell's Deoxyribonucleic acid, surrounded by a network of gristly intermediate filaments and enveloped in a double membrane called the nuclear envelope. The nuclear envelope separates the fluid within the nucleus, chosen the nucleoplasm, from the balance of the prison cell. The size of the nucleus depends on the size of the cell it is contained in, with a nucleus typically occupying nearly eight% of the full prison cell book.[1] The nucleus is the largest organelle in animal cells.[2] : 12 In mammalian cells, the boilerplate diameter of the nucleus is approximately vi micrometres (µm).[3]
Nuclear envelope and pores
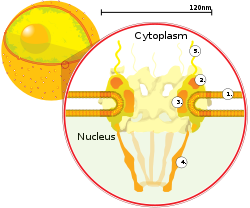
A cross department of a nuclear pore on the surface of the nuclear envelope (1). Other diagram labels show (2) the outer ring, (three) spokes, (4) basket, and (5) filaments.
The nuclear envelope consists of two membranes, an inner and an outer nuclear membrane.[iv] : 649 Together, these membranes serve to separate the cell's genetic cloth from the balance of the cell contents, and allow the nucleus to maintain an environment distinct from the rest of the cell. Despite their close apposition around much of the nucleus, the two membranes differ substantially in shape and contents. The inner membrane surrounds the nuclear content, providing its defining border.[2] : 14 Embedded within the inner membrane, various proteins bind the intermediate filaments that give the nucleus its structure.[4] : 649 The outer membrane encloses the inner membrane, and is continuous with the side by side endoplasmic reticulum membrane.[4] : 649 As function of the endoplasmic reticulum membrane, the outer nuclear membrane is studded with ribosomes that are actively translating proteins across membrane.[4] : 649 The space between the 2 membranes is called the perinuclear space, and is continuous with the endoplasmic reticulum lumen.[4] : 649
Nuclear pores, which provide aqueous channels through the envelope, are composed of multiple proteins, collectively referred to as nucleoporins. The pores are about threescore–lxxx one thousand thousand daltons in molecular weight and consist of around l (in yeast) to several hundred proteins (in vertebrates).[ii] : 622–4 The pores are 100 nm in total bore; however, the gap through which molecules freely lengthened is but about 9 nm broad, due to the presence of regulatory systems within the center of the pore. This size selectively allows the passage of small water-soluble molecules while preventing larger molecules, such as nucleic acids and larger proteins, from inappropriately entering or exiting the nucleus. These large molecules must be actively transported into the nucleus instead. The nucleus of a typical mammalian cell will take near 3000 to 4000 pores throughout its envelope,[five] each of which contains an eightfold-symmetric ring-shaped construction at a position where the inner and outer membranes fuse.[6] Attached to the band is a structure called the nuclear basket that extends into the nucleoplasm, and a series of filamentous extensions that reach into the cytoplasm. Both structures serve to mediate binding to nuclear ship proteins.[7] : 509–10
Most proteins, ribosomal subunits, and some RNAs are transported through the pore complexes in a process mediated by a family unit of ship factors known as karyopherins. Those karyopherins that mediate movement into the nucleus are besides called importins, whereas those that mediate movement out of the nucleus are chosen exportins. Most karyopherins interact directly with their cargo, although some use adaptor proteins.[8] Steroid hormones such as cortisol and aldosterone, also as other small lipid-soluble molecules involved in intercellular signaling, can diffuse through the prison cell membrane and into the cytoplasm, where they bind nuclear receptor proteins that are trafficked into the nucleus. There they serve as transcription factors when bound to their ligand; in the absence of a ligand, many such receptors office every bit histone deacetylases that repress gene expression.[7] : 488
Nuclear lamina
In fauna cells, two networks of intermediate filaments provide the nucleus with mechanical support: The nuclear lamina forms an organized meshwork on the internal confront of the envelope, while less organized support is provided on the cytosolic face of the envelope. Both systems provide structural support for the nuclear envelope and anchoring sites for chromosomes and nuclear pores.[ix]
The nuclear lamina is equanimous more often than not of lamin proteins. Like all proteins, lamins are synthesized in the cytoplasm and later transported to the nucleus interior, where they are assembled before existence incorporated into the existing network of nuclear lamina.[10] [eleven] Lamins found on the cytosolic face of the membrane, such as emerin and nesprin, demark to the cytoskeleton to provide structural support. Lamins are likewise found inside the nucleoplasm where they form another regular structure, known as the nucleoplasmic veil,[12] [13] that is visible using fluorescence microscopy. The actual function of the veil is non articulate, although it is excluded from the nucleolus and is present during interphase.[14] Lamin structures that make upward the veil, such equally LEM3, bind chromatin and disrupting their construction inhibits transcription of protein-coding genes.[15]
Like the components of other intermediate filaments, the lamin monomer contains an alpha-helical domain used by two monomers to coil around each other, forming a dimer structure chosen a coiled coil. Two of these dimer structures so bring together next, in an antiparallel arrangement, to course a tetramer called a protofilament. Eight of these protofilaments grade a lateral system that is twisted to form a ropelike filament. These filaments can be assembled or disassembled in a dynamic mode, significant that changes in the length of the filament depend on the competing rates of filament add-on and removal.[9]
Mutations in lamin genes leading to defects in filament assembly crusade a group of rare genetic disorders known as laminopathies. The most notable laminopathy is the family of diseases known equally progeria, which causes the appearance of premature aging in its sufferers. The verbal machinery past which the associated biochemical changes give rise to the aged phenotype is not well understood.[xvi]
Chromosomes
The cell nucleus contains the majority of the cell'due south genetic cloth in the form of multiple linear Dna molecules organized into structures called chromosomes. Each man cell contains roughly two meters of DNA.[7] : 405 During most of the cell cycle these are organized in a Dna-protein circuitous known as chromatin, and during prison cell division the chromatin tin exist seen to form the well-divers chromosomes familiar from a karyotype. A minor fraction of the cell's genes are located instead in the mitochondria.[7] : 438
At that place are two types of chromatin. Euchromatin is the less meaty Deoxyribonucleic acid form, and contains genes that are frequently expressed by the cell.[17] The other type, heterochromatin, is the more compact form, and contains Dna that is infrequently transcribed. This construction is further categorized into facultative heterochromatin, consisting of genes that are organized every bit heterochromatin just in certain cell types or at certain stages of development, and constitutive heterochromatin that consists of chromosome structural components such every bit telomeres and centromeres.[18] During interphase the chromatin organizes itself into discrete individual patches,[nineteen] called chromosome territories.[20] Active genes, which are generally found in the euchromatic region of the chromosome, tend to be located towards the chromosome'south territory purlieus.[21]
Antibodies to certain types of chromatin organization, in item, nucleosomes, have been associated with a number of autoimmune diseases, such as systemic lupus erythematosus.[22] These are known equally anti-nuclear antibodies (ANA) and have too been observed in concert with multiple sclerosis as part of general allowed arrangement dysfunction.[23]
Nucleolus

The nucleolus is the largest of the discrete densely stained, membraneless structures known as nuclear bodies establish in the nucleus. It forms around tandem repeats of rDNA, DNA coding for ribosomal RNA (rRNA). These regions are chosen nucleolar organizer regions (NOR). The master roles of the nucleolus are to synthesize rRNA and assemble ribosomes. The structural cohesion of the nucleolus depends on its activeness, as ribosomal assembly in the nucleolus results in the transient association of nucleolar components, facilitating further ribosomal assembly, and hence further association. This model is supported by observations that inactivation of rDNA results in intermingling of nucleolar structures.[24]
In the first step of ribosome associates, a protein called RNA polymerase I transcribes rDNA, which forms a large pre-rRNA precursor. This is cleaved into two large rRNA subunits – 5.8S, and 28S, and a minor rRNA subunit 18S.[iv] : 328 [25] The transcription, mail service-transcriptional processing, and assembly of rRNA occurs in the nucleolus, aided by modest nucleolar RNA (snoRNA) molecules, some of which are derived from spliced introns from messenger RNAs encoding genes related to ribosomal office. The assembled ribosomal subunits are the largest structures passed through the nuclear pores.[7] : 526
When observed under the electron microscope, the nucleolus tin can exist seen to consist of three distinguishable regions: the innermost fibrillar centers (FCs), surrounded past the dumbo fibrillar component (DFC) (that contains fibrillarin and nucleolin), which in turn is bordered past the granular component (GC) (that contains the protein nucleophosmin). Transcription of the rDNA occurs either in the FC or at the FC-DFC boundary, and, therefore, when rDNA transcription in the cell is increased, more FCs are detected. Near of the cleavage and modification of rRNAs occurs in the DFC, while the latter steps involving protein assembly onto the ribosomal subunits occur in the GC.[25]
Other nuclear bodies
| Construction name | Structure diameter | Ref. |
|---|---|---|
| Cajal bodies | 0.2–2.0 µm | [26] |
| Clastosomes | 0.ii-0.5 µm | [27] |
| PIKA | v µm | [28] |
| PML bodies | 0.ii–1.0 µm | [29] |
| Paraspeckles | 0.5–ane.0 µm | [xxx] |
| Speckles | xx–25 nm | [28] |
Besides the nucleolus, the nucleus contains a number of other nuclear bodies. These include Cajal bodies, gemini of Cajal bodies, polymorphic interphase karyosomal association (PIKA), promyelocytic leukaemia (PML) bodies, paraspeckles, and splicing speckles. Although picayune is known about a number of these domains, they are meaning in that they testify that the nucleoplasm is not a uniform mixture, but rather contains organized functional subdomains.[29]
Other subnuclear structures appear every bit part of aberrant affliction processes. For example, the presence of small intranuclear rods has been reported in some cases of nemaline myopathy. This condition typically results from mutations in actin, and the rods themselves consist of mutant actin as well as other cytoskeletal proteins.[31]
Cajal bodies and gems
A nucleus typically contains between i and 10 compact structures chosen Cajal bodies or coiled bodies (CB), whose diameter measures between 0.2 µm and 2.0 µm depending on the cell type and species.[26] When seen nether an electron microscope, they resemble balls of tangled thread[28] and are dense foci of distribution for the protein coilin.[32] CBs are involved in a number of different roles relating to RNA processing, specifically small nucleolar RNA (snoRNA) and small nuclear RNA (snRNA) maturation, and histone mRNA modification.[26]
Similar to Cajal bodies are Gemini of Cajal bodies, or gems, whose name is derived from the Gemini constellation in reference to their close "twin" relationship with CBs. Gems are similar in size and shape to CBs, and in fact are nearly duplicate under the microscope.[32] Unlike CBs, gems do non comprise small nuclear ribonucleoproteins (snRNPs), but practice contain a protein called survival of motor neuron (SMN) whose role relates to snRNP biogenesis. Gems are believed to assistance CBs in snRNP biogenesis,[33] though it has also been suggested from microscopy evidence that CBs and gems are unlike manifestations of the same structure.[32] Later ultrastructural studies have shown gems to be twins of Cajal bodies with the difference being in the coilin component; Cajal bodies are SMN positive and coilin positive, and gems are SMN positive and coilin negative.[34]
PIKA and PTF domains
PIKA domains, or polymorphic interphase karyosomal associations, were first described in microscopy studies in 1991. Their function remains unclear, though they were not thought to be associated with agile Dna replication, transcription, or RNA processing.[35] They have been found to oft acquaintance with discrete domains defined by dumbo localization of the transcription factor PTF, which promotes transcription of small nuclear RNA (snRNA).[36]
PML-nuclear bodies
Promyelocytic leukemia protein (PML-nuclear bodies) are spherical bodies found scattered throughout the nucleoplasm, measuring around 0.1–i.0 µm. They are known past a number of other names, including nuclear domain ten (ND10), Kremer bodies, and PML oncogenic domains.[37] PML-nuclear bodies are named later on one of their major components, the promyelocytic leukemia poly peptide (PML). They are oft seen in the nucleus in association with Cajal bodies and cleavage bodies.[29] Pml-/- mice, which are unable to create PML-nuclear bodies, develop ordinarily without obvious ill furnishings, showing that PML-nuclear bodies are not required for most essential biological processes.[38]
Splicing speckles
Speckles are subnuclear structures that are enriched in pre-messenger RNA splicing factors and are located in the interchromatin regions of the nucleoplasm of mammalian cells.[39] At the fluorescence-microscope level they announced as irregular, punctate structures, which vary in size and shape, and when examined past electron microscopy they are seen as clusters of interchromatin granules. Speckles are dynamic structures, and both their protein and RNA-protein components can wheel continuously between speckles and other nuclear locations, including active transcription sites. Speckles can work with p53 as enhancers of gene activeness to directly enhance the activeness of certain genes. Moreover, speckle-associating and not-associating p53 gene targets are functionally distinct.[40]
Studies on the composition, construction and behaviour of speckles accept provided a model for agreement the functional compartmentalization of the nucleus and the organization of the gene-expression mechanism[41] splicing snRNPs[42] [43] and other splicing proteins necessary for pre-mRNA processing.[41] Because of a prison cell's changing requirements, the limerick and location of these bodies changes according to mRNA transcription and regulation via phosphorylation of specific proteins.[44] The splicing speckles are too known every bit nuclear speckles (nuclear specks), splicing factor compartments (SF compartments), interchromatin granule clusters (IGCs), and B snurposomes.[45] B snurposomes are found in the amphibian oocyte nuclei and in Drosophila melanogaster embryos. B snurposomes appear alone or fastened to the Cajal bodies in the electron micrographs of the amphibian nuclei.[46] IGCs function as storage sites for the splicing factors.[47]
Paraspeckles
Discovered by Flim-flam et al. in 2002, paraspeckles are irregularly shaped compartments in the interchromatin infinite of the nucleus.[48] Showtime documented in HeLa cells, where in that location are more often than not 10–30 per nucleus,[49] paraspeckles are now known to also be in all human master cells, transformed cell lines, and tissue sections.[l] Their name is derived from their distribution in the nucleus; the "para" is short for parallel and the "speckles" refers to the splicing speckles to which they are always in shut proximity.[49]
Paraspeckles sequester nuclear proteins and RNA and thus appear to function as a molecular sponge[51] that is involved in the regulation of gene expression.[52] Furthermore, paraspeckles are dynamic structures that are contradistinct in response to changes in cellular metabolic activeness. They are transcription dependent[48] and in the absence of RNA Pol Ii transcription, the paraspeckle disappears and all of its associated protein components (PSP1, p54nrb, PSP2, CFI(m)68, and PSF) course a crescent shaped perinucleolar cap in the nucleolus. This phenomenon is demonstrated during the cell bike. In the cell cycle, paraspeckles are present during interphase and during all of mitosis except for telophase. During telophase, when the two daughter nuclei are formed, there is no RNA Political leader Two transcription so the protein components instead class a perinucleolar cap.[50]
Perichromatin fibrils
Perichromatin fibrils are visible only under electron microscope. They are located next to the transcriptionally active chromatin and are hypothesized to exist the sites of active pre-mRNA processing.[47]
Clastosomes
Clastosomes are small-scale nuclear bodies (0.2–0.v µm) described as having a thick band-shape due to the peripheral capsule around these bodies.[27] This name is derived from the Greek klastos, broken and soma, trunk.[27] Clastosomes are non typically present in normal cells, making them hard to observe. They form under loftier proteolytic conditions inside the nucleus and dethrone once there is a subtract in activity or if cells are treated with proteasome inhibitors.[27] [53] The scarcity of clastosomes in cells indicates that they are not required for proteasome function.[54] Osmotic stress has also been shown to cause the formation of clastosomes.[55] These nuclear bodies contain catalytic and regulatory subunits of the proteasome and its substrates, indicating that clastosomes are sites for degrading proteins.[54]
Role
The nucleus provides a site for genetic transcription that is segregated from the location of translation in the cytoplasm, allowing levels of gene regulation that are not available to prokaryotes. The main role of the jail cell nucleus is to control gene expression and mediate the replication of DNA during the cell cycle.[7] : 171
Cell compartmentalization
The nuclear envelope allows control of the nuclear contents, and separates them from the rest of the cytoplasm where necessary. This is of import for controlling processes on either side of the nuclear membrane: In almost cases where a cytoplasmic process needs to be restricted, a key participant is removed to the nucleus, where information technology interacts with transcription factors to downregulate the production of sure enzymes in the pathway. This regulatory mechanism occurs in the case of glycolysis, a cellular pathway for breaking down glucose to produce energy. Hexokinase is an enzyme responsible for the first the stride of glycolysis, forming glucose-vi-phosphate from glucose. At high concentrations of fructose-six-phosphate, a molecule made later from glucose-six-phosphate, a regulator protein removes hexokinase to the nucleus,[56] where it forms a transcriptional repressor complex with nuclear proteins to reduce the expression of genes involved in glycolysis.[57]
In guild to control which genes are being transcribed, the cell separates some transcription cistron proteins responsible for regulating cistron expression from physical access to the DNA until they are activated past other signaling pathways. This prevents even depression levels of inappropriate cistron expression. For example, in the case of NF-κB-controlled genes, which are involved in most inflammatory responses, transcription is induced in response to a signal pathway such as that initiated by the signaling molecule TNF-α, binds to a jail cell membrane receptor, resulting in the recruitment of signalling proteins, and eventually activating the transcription factor NF-κB. A nuclear localisation signal on the NF-κB protein allows information technology to be transported through the nuclear pore and into the nucleus, where it stimulates the transcription of the target genes.[ix]
The compartmentalization allows the cell to preclude translation of unspliced mRNA.[58] Eukaryotic mRNA contains introns that must be removed before beingness translated to produce functional proteins. The splicing is washed inside the nucleus before the mRNA can be accessed by ribosomes for translation. Without the nucleus, ribosomes would interpret newly transcribed (unprocessed) mRNA, resulting in malformed and nonfunctional proteins.[7] : 108–xv
Replication
The master function of the prison cell nucleus is to control gene expression and mediate the replication of Dna during the jail cell cycle.[7] : 171 Information technology has been found that replication happens in a localised style in the cell nucleus. In the S phase of interphase of the cell bike; replication takes place. Reverse to the traditional view of moving replication forks along stagnant Dna, a concept of replication factories emerged, which means replication forks are concentrated towards some immobilised 'factory' regions through which the template Deoxyribonucleic acid strands pass like conveyor belts.[59]
Cistron expression

A generic transcription manufactory during transcription, highlighting the possibility of transcribing more than than ane gene at a time. The diagram includes viii RNA polymerases however the number can vary depending on cell type. The image also includes transcription factors and a porous, protein cadre.
Cistron expression first involves transcription, in which DNA is used every bit a template to produce RNA. In the instance of genes encoding proteins, that RNA produced from this process is messenger RNA (mRNA), which then needs to be translated by ribosomes to form a protein. As ribosomes are located outside the nucleus, mRNA produced needs to be exported.[60]
Since the nucleus is the site of transcription, information technology too contains a variety of proteins that either direct mediate transcription or are involved in regulating the process. These proteins include helicases, which unwind the double-stranded Deoxyribonucleic acid molecule to facilitate admission to information technology, RNA polymerases, which bind to the Deoxyribonucleic acid promoter to synthesize the growing RNA molecule, topoisomerases, which change the amount of supercoiling in Deoxyribonucleic acid, helping it wind and unwind, as well as a large diversity of transcription factors that regulate expression.[61]
Processing of pre-mRNA
Newly synthesized mRNA molecules are known as primary transcripts or pre-mRNA. They must undergo post-transcriptional modification in the nucleus before being exported to the cytoplasm; mRNA that appears in the cytoplasm without these modifications is degraded rather than used for protein translation. The three master modifications are five' capping, 3' polyadenylation, and RNA splicing. While in the nucleus, pre-mRNA is associated with a variety of proteins in complexes known as heterogeneous ribonucleoprotein particles (hnRNPs). Addition of the 5' cap occurs co-transcriptionally and is the get-go pace in postal service-transcriptional modification. The iii' poly-adenine tail is only added after transcription is consummate.[7] : 509–18
RNA splicing, carried out by a circuitous called the spliceosome, is the process past which introns, or regions of DNA that do non code for protein, are removed from the pre-mRNA and the remaining exons continued to re-grade a single continuous molecule. This process commonly occurs later on v' capping and iii' polyadenylation simply tin can begin earlier synthesis is complete in transcripts with many exons.[7] : 494 Many pre-mRNAs can exist spliced in multiple means to produce different mature mRNAs that encode different poly peptide sequences. This procedure is known as alternative splicing, and allows production of a large multifariousness of proteins from a limited amount of DNA.[62]
Dynamics and regulation
Nuclear transport

The entry and go out of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus without regulation,[63] macromolecules such as RNA and proteins require association karyopherins called importins to enter the nucleus and exportins to get out. "Cargo" proteins that must be translocated from the cytoplasm to the nucleus contain brusque amino acid sequences known every bit nuclear localization signals, which are spring by importins, while those transported from the nucleus to the cytoplasm carry nuclear export signals bound past exportins. The power of importins and exportins to ship their cargo is regulated past GTPases, enzymes that hydrolyze the molecule guanosine triphosphate (GTP) to release energy. The fundamental GTPase in nuclear transport is Ran, which is jump to either GTP or Gross domestic product (guanosine diphosphate), depending on whether it is located in the nucleus or the cytoplasm. Whereas importins depend on RanGTP to dissociate from their cargo, exportins require RanGTP in social club to bind to their cargo.[8]
Nuclear import depends on the importin binding its cargo in the cytoplasm and carrying information technology through the nuclear pore into the nucleus. Inside the nucleus, RanGTP acts to separate the cargo from the importin, assuasive the importin to leave the nucleus and be reused. Nuclear export is similar, equally the exportin binds the cargo inside the nucleus in a process facilitated past RanGTP, exits through the nuclear pore, and separates from its cargo in the cytoplasm.[64]
Specialized consign proteins exist for translocation of mature mRNA and tRNA to the cytoplasm subsequently post-transcriptional modification is complete. This quality-control mechanism is important due to these molecules' central office in protein translation. Mis-expression of a poly peptide due to incomplete excision of exons or mis-incorporation of amino acids could have negative consequences for the prison cell; thus, incompletely modified RNA that reaches the cytoplasm is degraded rather than used in translation.[7]
Assembly and disassembly

During its lifetime, a nucleus may be broken downwards or destroyed, either in the process of cell sectionalisation or as a consequence of apoptosis (the process of programmed cell expiry). During these events, the structural components of the nucleus — the envelope and lamina — tin be systematically degraded. In most cells, the disassembly of the nuclear envelope marks the cease of the prophase of mitosis. However, this disassembly of the nucleus is not a universal feature of mitosis and does not occur in all cells. Some unicellular eukaryotes (east.g., yeasts) undergo and so-chosen closed mitosis, in which the nuclear envelope remains intact. In closed mitosis, the daughter chromosomes migrate to reverse poles of the nucleus, which then divides in two. The cells of higher eukaryotes, nonetheless, unremarkably undergo open mitosis, which is characterized past breakdown of the nuclear envelope. The daughter chromosomes then migrate to reverse poles of the mitotic spindle, and new nuclei reassemble around them.[7] : 854
At a certain signal during the cell cycle in open mitosis, the jail cell divides to course two cells. In order for this procedure to exist possible, each of the new daughter cells must have a full gear up of genes, a process requiring replication of the chromosomes as well as segregation of the separate sets. This occurs by the replicated chromosomes, the sister chromatids, attaching to microtubules, which in plow are attached to different centrosomes. The sis chromatids tin then be pulled to separate locations in the cell. In many cells, the centrosome is located in the cytoplasm, outside the nucleus; the microtubules would be unable to attach to the chromatids in the presence of the nuclear envelope.[65] Therefore, the early stages in the jail cell bike, beginning in prophase and until around prometaphase, the nuclear membrane is dismantled.[12] As well, during the same period, the nuclear lamina is likewise disassembled, a process regulated by phosphorylation of the lamins past protein kinases such as the CDC2 protein kinase.[66] Towards the end of the cell bike, the nuclear membrane is reformed, and effectually the same time, the nuclear lamina are reassembled past dephosphorylating the lamins.[66]
Even so, in dinoflagellates, the nuclear envelope remains intact, the centrosomes are located in the cytoplasm, and the microtubules come in contact with chromosomes, whose centromeric regions are incorporated into the nuclear envelope (the and then-called closed mitosis with extranuclear spindle). In many other protists (east.g., ciliates, sporozoans) and fungi, the centrosomes are intranuclear, and their nuclear envelope besides does not disassemble during cell partition.[67]
Apoptosis is a controlled process in which the cell's structural components are destroyed, resulting in death of the cell. Changes associated with apoptosis directly affect the nucleus and its contents, for example, in the condensation of chromatin and the disintegration of the nuclear envelope and lamina. The devastation of the lamin networks is controlled by specialized apoptotic proteases called caspases, which cleave the lamin proteins and, thus, degrade the nucleus' structural integrity. Lamin cleavage is sometimes used equally a laboratory indicator of caspase activity in assays for early apoptotic action.[12] Cells that limited mutant caspase-resistant lamins are scarce in nuclear changes related to apoptosis, suggesting that lamins play a function in initiating the events that pb to apoptotic degradation of the nucleus.[12] Inhibition of lamin assembly itself is an inducer of apoptosis.[68]
The nuclear envelope acts equally a barrier that prevents both DNA and RNA viruses from entering the nucleus. Some viruses crave access to proteins inside the nucleus in lodge to replicate and/or get together. DNA viruses, such as herpesvirus replicate and assemble in the cell nucleus, and exit by budding through the inner nuclear membrane. This process is accompanied by disassembly of the lamina on the nuclear face of the inner membrane.[12]
Initially, information technology has been suspected that immunoglobulins in general and autoantibodies in particular do not enter the nucleus. At present at that place is a body of evidence that under pathological atmospheric condition (east.g. lupus erythematosus) IgG tin enter the nucleus.[69]
Nuclei per jail cell
Most eukaryotic cell types commonly have a single nucleus, merely some take no nuclei, while others have several. This tin issue from normal development, every bit in the maturation of mammalian red blood cells, or from faulty cell partitioning.[70]
Anucleated cells

Human red blood cells, similar those of other mammals, lack nuclei. This occurs as a normal part of the cells' development.
An anucleated prison cell contains no nucleus and is, therefore, incapable of dividing to produce daughter cells. The best-known anucleated jail cell is the mammalian red blood prison cell, or erythrocyte, which also lacks other organelles such as mitochondria, and serves primarily as a transport vessel to ferry oxygen from the lungs to the trunk's tissues. Erythrocytes mature through erythropoiesis in the bone marrow, where they lose their nuclei, organelles, and ribosomes. The nucleus is expelled during the procedure of differentiation from an erythroblast to a reticulocyte, which is the immediate precursor of the mature erythrocyte.[71] The presence of mutagens may induce the release of some immature "micronucleated" erythrocytes into the bloodstream.[72] [73] Anucleated cells can also arise from flawed cell partition in which 1 girl lacks a nucleus and the other has two nuclei.
In flowering plants, this status occurs in sieve tube elements.[74]
Multinucleated cells
Multinucleated cells contain multiple nuclei. Near acantharean species of protozoa[75] and some fungi in mycorrhizae[76] have naturally multinucleated cells. Other examples include the intestinal parasites in the genus Giardia, which have ii nuclei per cell.[77] Ciliates have 2 kinds of nuclei in a single cell, a somatic macronucleus and a germline micronucleus.[78] In humans, skeletal musculus cells, also called myocytes and syncytium, become multinucleated during evolution; the resulting arrangement of nuclei near the periphery of the cells allows maximal intracellular space for myofibrils.[vii] Other multinucleate cells in the human are osteoclasts a type of bone cell. Multinucleated and binucleated cells can also exist abnormal in humans; for example, cells arising from the fusion of monocytes and macrophages, known as giant multinucleated cells, sometimes back-trail inflammation[79] and are also implicated in tumor formation.[80]
A number of dinoflagellates are known to have 2 nuclei. Unlike other multinucleated cells these nuclei contain two distinct lineages of DNA: ane from the dinoflagellate and the other from a symbiotic diatom.[81]
Evolution
As the major defining characteristic of the eukaryotic cell, the nucleus' evolutionary origin has been the field of study of much speculation. Four major hypotheses accept been proposed to explain the existence of the nucleus, although none have yet earned widespread back up.[82] [83] [84]
The first model known as the "syntrophic model" proposes that a symbiotic relationship betwixt the archaea and bacteria created the nucleus-containing eukaryotic cell. (Organisms of the Archaea and Leaner domain have no cell nucleus.[85]) It is hypothesized that the symbiosis originated when aboriginal archaea, similar to mod methanogenic archaea, invaded and lived inside bacteria similar to mod myxobacteria, eventually forming the early nucleus. This theory is analogous to the accepted theory for the origin of eukaryotic mitochondria and chloroplasts, which are thought to have adult from a like endosymbiotic relationship between proto-eukaryotes and aerobic bacteria.[86] I possibility is that the nuclear membrane arose as a new membrane system following the origin of mitochondria in an archaebacterial host.[87] The nuclear membrane may accept served to protect the genome from dissentious reactive oxygen species produced by the protomitochondria[88].The archaeal origin of the nucleus is supported by observations that archaea and eukarya have similar genes for certain proteins, including histones. Observations that myxobacteria are motile, tin can course multicellular complexes, and possess kinases and G proteins like to eukarya, support a bacterial origin for the eukaryotic cell.[89]
A second model proposes that proto-eukaryotic cells evolved from bacteria without an endosymbiotic stage. This model is based on the existence of modern Planctomycetota leaner that possess a nuclear construction with primitive pores and other compartmentalized membrane structures.[xc] A similar proposal states that a eukaryote-like jail cell, the chronocyte, evolved offset and phagocytosed archaea and leaner to generate the nucleus and the eukaryotic jail cell.[91]
The most controversial model, known equally viral eukaryogenesis, posits that the membrane-spring nucleus, forth with other eukaryotic features, originated from the infection of a prokaryote by a virus. The suggestion is based on similarities betwixt eukaryotes and viruses such as linear DNA strands, mRNA capping, and tight binding to proteins (analogizing histones to viral envelopes). I version of the proposal suggests that the nucleus evolved in concert with phagocytosis to form an early cellular "predator".[92] Some other variant proposes that eukaryotes originated from early archaea infected by poxviruses, on the basis of observed similarity between the DNA polymerases in mod poxviruses and eukaryotes.[93] [94] It has been suggested that the unresolved question of the evolution of sex could be related to the viral eukaryogenesis hypothesis.[95]
A more recent proposal, the exomembrane hypothesis, suggests that the nucleus instead originated from a unmarried ancestral cell that evolved a 2d exterior cell membrane; the interior membrane enclosing the original cell so became the nuclear membrane and evolved increasingly elaborate pore structures for passage of internally synthesized cellular components such as ribosomal subunits.[96]
History


The nucleus was the get-go organelle to exist discovered. What is near probable the oldest preserved cartoon dates back to the early microscopist Antonie van Leeuwenhoek (1632–1723). He observed a "lumen", the nucleus, in the red blood cells of salmon.[97] Dissimilar mammalian red claret cells, those of other vertebrates still contain nuclei.[98]
The nucleus was likewise described by Franz Bauer in 1804[99] and in more than detail in 1831 by Scottish botanist Robert Brown in a talk at the Linnean Club of London. Brown was studying orchids under the microscope when he observed an opaque surface area, which he chosen the "areola" or "nucleus", in the cells of the flower's outer layer.[100] He did not propose a potential function.
In 1838, Matthias Schleiden proposed that the nucleus plays a role in generating cells, thus he introduced the proper noun "cytoblast" ("cell builder"). He believed that he had observed new cells assembling around "cytoblasts". Franz Meyen was a strong opponent of this view, having already described cells multiplying by division and assertive that many cells would have no nuclei. The thought that cells can be generated de novo, by the "cytoblast" or otherwise, contradicted work by Robert Remak (1852) and Rudolf Virchow (1855) who decisively propagated the new paradigm that cells are generated solely by cells (" Omnis cellula e cellula "). The function of the nucleus remained unclear.[101]
Between 1877 and 1878, Oscar Hertwig published several studies on the fertilization of ocean urchin eggs, showing that the nucleus of the sperm enters the oocyte and fuses with its nucleus. This was the beginning time it was suggested that an individual develops from a (single) nucleated cell. This was in contradiction to Ernst Haeckel's theory that the complete phylogeny of a species would exist repeated during embryonic development, including generation of the first nucleated prison cell from a "monerula", a structureless mass of primordial protoplasm ("Urschleim"). Therefore, the necessity of the sperm nucleus for fertilization was discussed for quite some time. However, Hertwig confirmed his observation in other animal groups, including amphibians and molluscs. Eduard Strasburger produced the aforementioned results for plants in 1884. This paved the way to assign the nucleus an important role in heredity. In 1873, August Weismann postulated the equivalence of the maternal and paternal germ cells for heredity. The function of the nucleus as carrier of genetic information became clear only later, after mitosis was discovered and the Mendelian rules were rediscovered at the get-go of the 20th century; the chromosome theory of heredity was therefore developed.[101]
See besides
- Nucleus (neuroanatomy)
- Nucleoid
- Nucleomorph
References
- ^ Cantwell H, Nurse P (2019). "Unravelling nuclear size control". Current Genetics. Springer. 65 (6): 1282. doi:10.1007/s00294-019-00999-3. PMC6820586. PMID 31147736.
- ^ a b c Lodish HF, Berk A, Kaiser C, Krieger M, Bretscher A, Ploegh H, et al. (2016). Molecular Cell Biology (Eighth ed.). New York: W.H. Freeman. ISBN978-1-4641-8339-3.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the prison cell (quaternary ed.). New York: Garland Science. p. 197. ISBN978-0-8153-4072-0.
- ^ a b c d e f Alberts B, Johnson A, Lewis J, Morgan D, Raff One thousand, Roberts Chiliad, Walter P (2015). Molecular Biology of the Prison cell (6 ed.). New York: Garland Scientific discipline.
- ^ Rhoades R, Pflanzer R, eds. (1996). "Ch3". Human Physiology (3rd ed.). Saunders College Publishing.
- ^ Shulga N, Mosammaparast Due north, Wozniak R, Goldfarb DS (May 2000). "Yeast nucleoporins involved in passive nuclear envelope permeability". Primary. The Periodical of Cell Biological science. 149 (five): 1027–38. doi:10.1083/jcb.149.5.1027. PMC2174828. PMID 10831607.
- ^ a b c d eastward f k h i j k fifty k Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger Yard, Scott MP, Zipursky SL, Darnell J (2004). Molecular Cell Biology (fifth ed.). New York: WH Freeman. ISBN978-0-7167-2672-2.
- ^ a b Pemberton LF, Paschal BM (March 2005). "Mechanisms of receptor-mediated nuclear import and nuclear consign". Review. Traffic. 6 (iii): 187–98. doi:10.1111/j.1600-0854.2005.00270.x. PMID 15702987. S2CID 172279.
- ^ a b c Alberts B, Johnson A, Lewis J, Raff K, Roberts K, Walter P, eds. (2002). "Affiliate 4: Deoxyribonucleic acid and Chromosomes". Molecular Biology of the Cell (quaternary ed.). New York: Garland Science. pp. 191–234. ISBN978-0-8153-4072-0.
- ^ Stuurman Due north, Heins S, Aebi U (1998). "Nuclear lamins: their structure, assembly, and interactions". Review. Journal of Structural Biological science. 122 (1–ii): 42–66. doi:x.1006/jsbi.1998.3987. PMID 9724605.
- ^ Goldman AE, Moir RD, Montag-Lowy M, Stewart 1000, Goldman RD (Nov 1992). "Pathway of incorporation of microinjected lamin A into the nuclear envelope". Primary. The Journal of Cell Biology. 119 (4): 725–35. doi:ten.1083/jcb.119.4.725. PMC2289687. PMID 1429833.
- ^ a b c d e Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (March 2002). "Nuclear lamins: building blocks of nuclear architecture". Review. Genes & Development. 16 (5): 533–47. doi:ten.1101/gad.960502. PMID 11877373.
- ^ Broers JL, Ramaekers FC (2004). "Dynamics of nuclear lamina assembly and disassembly". Review. Symposia of the Club for Experimental Biological science (56): 177–92. ISBN9781134279838. PMID 15565881.
- ^ Moir RD, Yoon M, Khuon S, Goldman RD (December 2000). "Nuclear lamins A and B1: different pathways of associates during nuclear envelope formation in living cells". Principal. The Journal of Cell Biological science. 151 (half dozen): 1155–68. doi:10.1083/jcb.151.6.1155. PMC2190592. PMID 11121432.
- ^ Spann TP, Goldman AE, Wang C, Huang South, Goldman RD (Feb 2002). "Amending of nuclear lamin organization inhibits RNA polymerase Two-dependent transcription". Primary. The Journal of Cell Biology. 156 (iv): 603–8. doi:x.1083/jcb.200112047. PMC2174089. PMID 11854306.
- ^ Mounkes LC, Stewart CL (June 2004). "Crumbling and nuclear organization: lamins and progeria". Review. Current Opinion in Cell Biology. sixteen (iii): 322–7. doi:10.1016/j.ceb.2004.03.009. PMID 15145358.
- ^ Ehrenhofer-Murray AE (June 2004). "Chromatin dynamics at Dna replication, transcription and repair". Review. European Periodical of Biochemistry. 271 (12): 2335–49. doi:x.1111/j.1432-1033.2004.04162.10. PMID 15182349.
- ^ Grigoryev SA, Bulynko YA, Popova EY (2006). "The end adjusts the means: heterochromatin remodelling during final cell differentiation". Review. Chromosome Enquiry. 14 (1): 53–69. doi:10.1007/s10577-005-1021-6. PMID 16506096. S2CID 6040822.
- ^ Schardin Thou, Cremer T, Hager Hd, Lang Thousand (December 1985). "Specific staining of human chromosomes in Chinese hamster 10 man hybrid jail cell lines demonstrates interphase chromosome territories" (PDF). Primary. Human Genetics. 71 (4): 281–seven. doi:10.1007/BF00388452. PMID 2416668. S2CID 9261461.
- ^ Lamond AI, Earnshaw WC (April 1998). "Structure and function in the nucleus" (PDF). Review. Science. 280 (5363): 547–53. CiteSeerX10.one.1.323.5543. doi:10.1126/science.280.5363.547. PMID 9554838.
- ^ Kurz A, Lampel S, Nickolenko JE, Bradl J, Benner A, Zirbel RM, et al. (Dec 1996). "Active and inactive genes localize preferentially in the periphery of chromosome territories". Master. The Periodical of Cell Biology. 135 (5): 1195–205. doi:ten.1083/jcb.135.five.1195. PMC2121085. PMID 8947544. Archived from the original on 29 September 2007.
- ^ Rothfield NF, Stollar BD (Nov 1967). "The relation of immunoglobulin class, pattern of anti-nuclear antibody, and complement-fixing antibodies to Deoxyribonucleic acid in sera from patients with systemic lupus erythematosus". Master. The Journal of Clinical Investigation. 46 (11): 1785–94. doi:ten.1172/JCI105669. PMC292929. PMID 4168731.
- ^ Barned S, Goodman AD, Mattson DH (Feb 1995). "Frequency of anti-nuclear antibodies in multiple sclerosis". Primary. Neurology. 45 (2): 384–5. doi:ten.1212/WNL.45.ii.384. PMID 7854544. S2CID 30482028.
- ^ Hernandez-Verdun D (Jan 2006). "Nucleolus: from structure to dynamics". Review. Histochemistry and Cell Biology. 125 (one–2): 127–37. doi:10.1007/s00418-005-0046-4. PMID 16328431. S2CID 20769260.
- ^ a b Lamond AI, Sleeman JE (October 2003). "Nuclear substructure and dynamics". Review. Current Biology. xiii (21): R825-8. doi:x.1016/j.cub.2003.10.012. PMID 14588256. S2CID 16865665.
- ^ a b c Cioce Thousand, Lamond AI (2005). "Cajal bodies: a long history of discovery". Review. Annual Review of Cell and Developmental Biology. 21: 105–31. doi:10.1146/annurev.cellbio.20.010403.103738. PMID 16212489. S2CID 8807316.
- ^ a b c d Lafarga One thousand, Berciano MT, Pena Eastward, Mayo I, Castaño JG, Bohmann D, et al. (August 2002). "Clastosome: a subtype of nuclear body enriched in 19S and 20S proteasomes, ubiquitin, and poly peptide substrates of proteasome". Primary. Molecular Biological science of the Cell. xiii (8): 2771–82. CiteSeerXx.1.1.321.6138. doi:10.1091/mbc.e02-03-0122. PMC117941. PMID 12181345.
- ^ a b c Pollard TD, Earnshaw WC (2004). Cell Biology . Philadelphia: Saunders. ISBN978-0-7216-3360-2.
- ^ a b c Dundr M, Misteli T (June 2001). "Functional architecture in the jail cell nucleus". Review. The Biochemical Journal. 356 (Pt 2): 297–310. doi:10.1042/0264-6021:3560297. PMC1221839. PMID 11368755.
- ^ Bond CS, Play a trick on AH (September 2009). "Paraspeckles: nuclear bodies built on long noncoding RNA". Review. The Periodical of Prison cell Biology. 186 (5): 637–44. doi:ten.1083/jcb.200906113. PMC2742191. PMID 19720872.
- ^ Goebel HH, Warlo I (January 1997). "Nemaline myopathy with intranuclear rods--intranuclear rod myopathy". Review. Neuromuscular Disorders. vii (1): thirteen–ix. doi:ten.1016/S0960-8966(96)00404-X. PMID 9132135. S2CID 29584217.
- ^ a b c Matera AG, Frey MR (Baronial 1998). "Coiled bodies and gems: Janus or gemini?". Review. American Journal of Human Genetics. 63 (2): 317–21. doi:ten.1086/301992. PMC1377332. PMID 9683623.
- ^ Matera AG (August 1998). "Of coiled bodies, gems, and salmon". Review. Periodical of Cellular Biochemistry. 70 (2): 181–92. doi:10.1002/(sici)1097-4644(19980801)70:2<181::help-jcb4>3.0.co;2-thou. PMID 9671224. S2CID 44941483.
- ^ Saunders WS, Cooke CA, Earnshaw WC (November 1991). "Compartmentalization within the nucleus: discovery of a novel subnuclear region". Chief. The Journal of Jail cell Biology. 115 (4): 919–31. doi:10.1083/jcb.115.4.919. PMC2289954. PMID 1955462.
- ^ Pombo A, Cuello P, Schul W, Yoon JB, Roeder RG, Cook PR, Murphy S (March 1998). "Regional and temporal specialization in the nucleus: a transcriptionally-active nuclear domain rich in PTF, Oct1 and PIKA antigens assembly with specific chromosomes early on in the jail cell cycle". Main. The EMBO Journal. 17 (six): 1768–78. doi:10.1093/emboj/17.6.1768. PMC1170524. PMID 9501098.
- ^ Zimber A, Nguyen QD, Gespach C (October 2004). "Nuclear bodies and compartments: functional roles and cellular signalling in health and illness". Review. Cellular Signalling. 16 (10): 1085–104. doi:10.1016/j.cellsig.2004.03.020. PMID 15240004.
- ^ Lallemand-Breitenbach V, de Thé H (May 2010). "PML nuclear bodies". Review. Cold Spring Harbor Perspectives in Biology. 2 (5): a000661. doi:ten.1101/cshperspect.a000661. PMC2857171. PMID 20452955.
- ^ Spector DL, Lamond AI (February 2011). "Nuclear speckles". Review. Cold Bound Harbor Perspectives in Biology. 3 (2): a000646. doi:10.1101/cshperspect.a000646. PMC3039535. PMID 20926517.
- ^ Alexander KA, Coté A, Nguyen SC, Zhang 50, Berger SL (March 2021). "p53 mediates target factor clan with nuclear speckles for amplified RNA expression". Main. Molecular Cell. 81 (8): S1097-2765(21)00174-X. doi:10.1016/j.molcel.2021.03.006. PMC8830378. PMID 33823140. S2CID 233172170.
- ^ a b Lamond AI, Spector DL (August 2003). "Nuclear speckles: a model for nuclear organelles". Review. Nature Reviews. Molecular Cell Biology. four (8): 605–12. doi:10.1038/nrm1172. PMID 12923522. S2CID 6439413.
- ^ Tripathi 1000, Parnaik VK (September 2008). "Differential dynamics of splicing factor SC35 during the jail cell cycle" (PDF). Master. Periodical of Biosciences. 33 (3): 345–54. doi:ten.1007/s12038-008-0054-3. PMID 19005234. S2CID 6332495. Archived (PDF) from the original on 15 Nov 2011.
- ^ Tripathi One thousand, Parnaik VK (September 2008). "Differential dynamics of splicing cistron SC35 during the cell cycle". Primary. Periodical of Biosciences. 33 (3): 345–54. doi:10.1007/s12038-008-0054-3. PMID 19005234. S2CID 6332495.
- ^ Handwerger KE, Gall JG (January 2006). "Subnuclear organelles: new insights into form and function". Review. Trends in Cell Biology. 16 (1): 19–26. doi:10.1016/j.tcb.2005.11.005. PMID 16325406.
- ^ "Cellular component Nucleus speckle". UniProt: UniProtKB. Retrieved 30 August 2013.
- ^ Gall JG, Bellini M, Wu Z, Murphy C (December 1999). "Associates of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes". Principal. Molecular Biology of the Prison cell. x (12): 4385–402. doi:10.1091/mbc.10.12.4385. PMC25765. PMID 10588665.
- ^ a b Matera AG, Terns RM, Terns MP (March 2007). "Not-coding RNAs: lessons from the pocket-size nuclear and small nucleolar RNAs". Review. Nature Reviews. Molecular Cell Biology. 8 (3): 209–20. doi:10.1038/nrm2124. PMID 17318225. S2CID 30268055.
- ^ a b Fox AH, Lamond AI (July 2010). "Paraspeckles". Review. Cold Jump Harbor Perspectives in Biological science. 2 (7): a000687. doi:10.1101/cshperspect.a000687. PMC2890200. PMID 20573717.
- ^ a b Fox A, Bickmore West (2004). "Nuclear Compartments: Paraspeckles". Nuclear Poly peptide Database. Archived from the original on ten September 2008. Retrieved six March 2007.
- ^ a b Fox AH, Bail CS, Lamond AI (November 2005). "P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner". Principal. Molecular Biological science of the Cell. sixteen (11): 5304–15. doi:10.1091/mbc.E05-06-0587. PMC1266428. PMID 16148043.
- ^ Nakagawa S, Yamazaki T, Hirose T (October 2018). "Molecular dissection of nuclear paraspeckles: towards understanding the emerging globe of the RNP milieu". Review. Open Biology. 8 (10): 180150. doi:10.1098/rsob.180150. PMC6223218. PMID 30355755.
- ^ Pisani G, Baron B (December 2019). "Nuclear paraspeckles function in mediating gene regulatory and apoptotic pathways". Review. Non-Coding RNA Inquiry. 4 (4): 128–134. doi:10.1016/j.ncrna.2019.11.002. PMC7012776. PMID 32072080.
- ^ Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, et al. (October 2007). "LPS-induced downwards-regulation of signal regulatory poly peptide {blastoff} contributes to innate immune activation in macrophages". Primary. The Journal of Experimental Medicine. 204 (eleven): 2719–31. doi:10.1084/jem.20062611. PMC2118489. PMID 17954568.
- ^ a b Carmo-Fonseca M, Berciano MT, Lafarga G (September 2010). "Orphan nuclear bodies". Review. Common cold Spring Harbor Perspectives in Biological science. 2 (9): a000703. doi:10.1101/cshperspect.a000703. PMC2926751. PMID 20610547.
- ^ Sampuda KM, Riley One thousand, Boyd 50 (April 2017). "Stress induced nuclear granules class in response to accumulation of misfolded proteins in Caenorhabditis elegans". Primary. BMC Prison cell Biological science. 18 (1): xviii. doi:10.1186/s12860-017-0136-x. PMC5395811. PMID 28424053.
- ^ Lehninger AL, Nelson DL, Cox MM (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. ISBN978-1-57259-931-iv.
- ^ Moreno F, Ahuatzi D, Riera A, Palomino CA, Herrero P (February 2005). "Glucose sensing through the Hxk2-dependent signalling pathway". Primary. Biochemical Social club Transactions. 33 (Pt 1): 265–8. doi:x.1042/BST0330265. PMID 15667322. S2CID 20647022.
- ^ Görlich D, Kutay U (1999). "Ship between the jail cell nucleus and the cytoplasm". Review. Annual Review of Cell and Developmental Biological science. fifteen (1): 607–60. doi:10.1146/annurev.cellbio.15.1.607. PMID 10611974.
- ^ Hozák P, Cook PR (Feb 1994). "Replication factories". Review. Trends in Cell Biology. iv (2): 48–52. doi:x.1016/0962-8924(94)90009-4. PMID 14731866.
- ^ Nierhaus KH, Wilson DN (2004). Protein Synthesis and Ribosome Structure: Translating the Genome. Wiley-VCH. ISBN978-3-527-30638-ane.
- ^ Nicolini CA (1997). Genome Structure and Function: From Chromosomes Characterization to Genes Technology. Springer. ISBN978-0-7923-4565-7.
- ^ Black DL (2003). "Mechanisms of alternative pre-messenger RNA splicing" (PDF). Review. Annual Review of Biochemistry. 72 (1): 291–336. doi:10.1146/annurev.biochem.72.121801.161720. PMID 12626338. S2CID 23576288.
- ^ Watson JD, Bakery TA, Bell SP, Gann A, Levine Chiliad, Losick R (2004). "Ch9–x". Molecular Biology of the Gene (fifth ed.). Peason Benjamin Cummings; CSHL Press. ISBN978-0-8053-9603-4.
- ^ Cavazza T, Vernos I (2015). "The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond". Review. Frontiers in Cell and Developmental Biology. 3: 82. doi:10.3389/fcell.2015.00082. PMC4707252. PMID 26793706.
- ^ Lippincott-Schwartz J (March 2002). "Prison cell biology: ripping upwards the nuclear envelope". Commentary. Nature. 416 (6876): 31–ii. Bibcode:2002Natur.416...31L. doi:10.1038/416031a. PMID 11882878. S2CID 4431000.
- ^ a b Boulikas T (1995). "Phosphorylation of transcription factors and control of the cell wheel". Review. Critical Reviews in Eukaryotic Gene Expression. 5 (1): 1–77. PMID 7549180.
- ^ Boettcher B, Barral Y (2013). "The cell biology of open and airtight mitosis". Review. Nucleus. Austin, Tex. iv (3): 160–5. doi:10.4161/nucl.24676. PMC3720745. PMID 23644379.
- ^ Steen RL, Collas P (April 2001). "Mistargeting of B-type lamins at the finish of mitosis: implications on cell survival and regulation of lamins A/C expression". Principal. The Periodical of Cell Biology. 153 (3): 621–6. doi:10.1083/jcb.153.3.621. PMC2190567. PMID 11331311.
- ^ Böhm I (Nov 2007). "IgG deposits can exist detected in cell nuclei of patients with both lupus erythematosus and malignancy". Principal. Clinical Rheumatology. 26 (11): 1877–82. doi:x.1007/s10067-007-0597-y. PMID 17364135. S2CID 44879431.
- ^ Ressel Fifty (2017). "Nuclear Morphologies". Normal cell morphology in canine and feline cytology: an identification guide. Hoboken, NJ: John Wiley & Sons. p. half dozen. ISBN978-1-119-27891-7.
- ^ Skutelsky Eastward, Danon D (June 1970). "Comparative report of nuclear expulsion from the late erythroblast and cytokinesis". Master. Experimental Cell Research. lx (3): 427–36. doi:10.1016/0014-4827(70)90536-vii. PMID 5422968.
- ^ Torous DK, Dertinger SD, Hall NE, Tometsko CR (February 2000). "Enumeration of micronucleated reticulocytes in rat peripheral blood: a flow cytometric study". Main. Mutation Enquiry. 465 (1–2): 91–ix. doi:10.1016/S1383-5718(99)00216-eight. PMID 10708974.
- ^ Hutter KJ, Stöhr 1000 (1982). "Rapid detection of mutagen induced micronucleated erythrocytes by menses cytometry". Master. Histochemistry. 75 (3): 353–62. doi:ten.1007/bf00496738. PMID 7141888. S2CID 28973947.
- ^ Ham BK, Lucas WJ (April 2014). "The angiosperm phloem sieve tube system: a part in mediating traits important to modernistic agriculture". Periodical of Experimental Phytology. 65 (seven): 1799–816. doi:10.1093/jxb/ert417. PMID 24368503.
- ^ Zettler LA, Sogin ML, Caron DA (October 1997). "Phylogenetic relationships between the Acantharea and the Polycystinea: a molecular perspective on Haeckel'south Radiolaria". Primary. Proceedings of the National Academy of Sciences of the United States of America. 94 (21): 11411–vi. Bibcode:1997PNAS...9411411A. doi:10.1073/pnas.94.21.11411. PMC23483. PMID 9326623.
- ^ Horton TR (2006). "The number of nuclei in basidiospores of 63 species of ectomycorrhizal Homobasidiomycetes". Principal. Mycologia. 98 (ii): 233–8. doi:10.3852/mycologia.98.two.233. PMID 16894968.
- ^ Adam RD (December 1991). "The biology of Giardia spp". Review. Microbiological Reviews. 55 (4): 706–32. doi:10.1128/MMBR.55.4.706-732.1991. PMC372844. PMID 1779932.
- ^ Vogt A, Goldman AD, Mochizuki K, Landweber LF (one August 2013). "Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements". PLOS Genetics. ix (8): e1003659. doi:10.1371/journal.pgen.1003659. PMC3731211. PMID 23935529.
- ^ McInnes A, Rennick DM (February 1988). "Interleukin iv induces cultured monocytes/macrophages to grade giant multinucleated cells". Primary. The Journal of Experimental Medicine. 167 (ii): 598–611. doi:10.1084/jem.167.2.598. PMC2188835. PMID 3258008.
- ^ Goldring SR, Roelke MS, Petrison KK, Bhan AK (February 1987). "Human giant cell tumors of bone identification and label of cell types". Primary. The Periodical of Clinical Investigation. 79 (ii): 483–91. doi:ten.1172/JCI112838. PMC424109. PMID 3027126.
- ^ Imanian B, Pombert JF, Dorrell RG, Burki F, Keeling PJ (2012). "Tertiary endosymbiosis in two dinotoms has generated little change in the mitochondrial genomes of their dinoflagellate hosts and diatom endosymbionts". Primary. PLOS ONE. 7 (8): e43763. Bibcode:2012PLoSO...743763I. doi:10.1371/journal.pone.0043763. PMC3423374. PMID 22916303.
- ^ Pennisi E (August 2004). "Evolutionary biology. The birth of the nucleus". News. Science. 305 (5685): 766–8. doi:x.1126/science.305.5685.766. PMID 15297641. S2CID 83769250.
- ^ Devos DP, Gräf R, Field MC (June 2014). "Evolution of the nucleus". Review. Current Opinion in Cell Biology. 28: 8–fifteen. doi:10.1016/j.ceb.2014.01.004. PMC4071446. PMID 24508984.
- ^ López-García P, Moreira D (November 2015). "Open Questions on the Origin of Eukaryotes". Review. Trends in Ecology & Development. 30 (11): 697–708. doi:10.1016/j.tree.2015.09.005. PMC4640172. PMID 26455774.
- ^ Hogan CM (2010). "Archaea". In Monosson E, Cleveland C (eds.). Encyclopedia of Earth. Washington, DC.: National Quango for Science and the Surround. Archived from the original on 11 May 2011.
- ^ Margulis L (1981). Symbiosis in Prison cell Evolution. San Francisco: W. H. Freeman and Company. pp. 206–227. ISBN978-0-7167-1256-5.
- ^ Martin Due west (December 2005). "Archaebacteria (Archaea) and the origin of the eukaryotic nucleus". Curr Opin Microbiol. 8 (vi): 630–vii. doi:ten.1016/j.mib.2005.10.004. PMID 16242992.
- ^ Bernstein, H., Bernstein, C. (2017). Sexual Advice in Archaea, the Precursor to Eukaryotic Meiosis. In: Witzany, Grand. (eds) Biocommunication of Archaea. Springer, Cham. https://doi.org/x.1007/978-3-319-65536-9_7
- ^ López-García P, Moreira D (May 2006). "Selective forces for the origin of the eukaryotic nucleus". Review. BioEssays. 28 (v): 525–33. doi:10.1002/bies.20413. PMID 16615090.
- ^ Fuerst JA (2005). "Intracellular compartmentation in planctomycetes". Review. Annual Review of Microbiology. 59: 299–328. doi:10.1146/annurev.micro.59.030804.121258. PMID 15910279.
- ^ Hartman H, Fedorov A (Feb 2002). "The origin of the eukaryotic cell: a genomic investigation". Primary. Proceedings of the National Academy of Sciences of the United states of america of America. 99 (3): 1420–5. Bibcode:2002PNAS...99.1420H. doi:ten.1073/pnas.032658599. PMC122206. PMID 11805300.
- ^ Bell PJ (September 2001). "Viral eukaryogenesis: was the antecedent of the nucleus a complex Deoxyribonucleic acid virus?". Annotate. Journal of Molecular Evolution. 53 (iii): 251–six. Bibcode:2001JMolE..53..251L. doi:10.1007/s002390010215. PMID 11523012. S2CID 20542871.
- ^ Takemura M (May 2001). "Poxviruses and the origin of the eukaryotic nucleus". Chief. Journal of Molecular Evolution. 52 (5): 419–25. Bibcode:2001JMolE..52..419T. doi:10.1007/s002390010171. PMID 11443345. S2CID 21200827.
- ^ Villarreal LP, DeFilippis VR (August 2000). "A hypothesis for Dna viruses equally the origin of eukaryotic replication proteins". Chief. Journal of Virology. 74 (15): 7079–84. doi:10.1128/JVI.74.fifteen.7079-7084.2000. PMC112226. PMID 10888648.
- ^ Bong PJ (November 2006). "Sex activity and the eukaryotic jail cell wheel is consistent with a viral ancestry for the eukaryotic nucleus". Principal. Journal of Theoretical Biology. 243 (one): 54–63. Bibcode:2006JThBi.243...54B. doi:10.1016/j.jtbi.2006.05.015. PMID 16846615.
- ^ de Roos AD (2006). "The origin of the eukaryotic jail cell based on conservation of existing interfaces". Primary. Artificial Life. 12 (4): 513–23. doi:10.1162/artl.2006.12.4.513. PMID 16953783. S2CID 5963228.
- ^ Van Leeuwenhoek A. Opera Omnia, seu Arcana Naturae ope exactissimorum Microscopiorum detecta, experimentis variis comprobata, Epistolis ad varios illustres viros J. Arnold et Delphis, A. Beman, Lugdinum Batavorum [The Works of, or arcana of nature past ways of exactissimorum microscopes had been detected and confirmed by a variety of experiments, the Epistles to the various illustrious men of valor J. Arnold and Delphi, A. Beman, Lugdina York 1719-1730] (in Latin). Cited in Gerlach D (2009). Geschichte der Mikroskopie. Frankfurt am Chief, Federal republic of germany: Verlag Harri Deutsch. ISBN978-three-8171-1781-9.
- ^ Cohen WD (1982). "The cytomorphic system of anucleate non-mammalian erythrocytes". Protoplasma. 113: 23–32. doi:10.1007/BF01283036. S2CID 41287948.
- ^ Harris H (1999). The Birth of the Cell . New Haven: Yale University Press. ISBN978-0-300-07384-three.
- ^ Chocolate-brown R (1866). "On the Organs and Mode of Fecundation of Orchidex and Asclepiadea". Miscellaneous Botanical Works I: 511–514.
- ^ a b Cremer T (1985). Von der Zellenlehre zur Chromosomentheorie. Berlin, Heidelberg, New York, Tokyo: Springer Verlag. ISBN978-3-540-13987-4. Online Version here
Further reading
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP (March 2002). "Nuclear lamins: edifice blocks of nuclear architecture". Genes & Evolution. 16 (5): 533–47. doi:10.1101/gad.960502. PMID 11877373.
- A review article virtually nuclear lamins, explaining their structure and diverse roles
- Görlich D, Kutay U (1999). "Ship betwixt the cell nucleus and the cytoplasm". Annual Review of Cell and Developmental Biology. 15: 607–60. doi:10.1146/annurev.cellbio.fifteen.one.607. PMID 10611974.
- A review article about nuclear transport, explains the principles of the mechanism, and the various transport pathways
- Lamond AI, Earnshaw WC (April 1998). "Structure and function in the nucleus" (PDF). Scientific discipline. 280 (5363): 547–53. CiteSeerX10.1.one.323.5543. doi:x.1126/science.280.5363.547. PMID 9554838.
- A review article virtually the nucleus, explaining the structure of chromosomes within the organelle, and describing the nucleolus and other subnuclear bodies
- Pennisi E (August 2004). "Evolutionary biology. The nascency of the nucleus". Science. 305 (5685): 766–viii. doi:x.1126/scientific discipline.305.5685.766. PMID 15297641. S2CID 83769250.
- A review article about the evolution of the nucleus, explaining a number of different theories
- Pollard TD, Earnshaw WC (2004). Cell Biological science. Philadelphia: Saunders. ISBN978-0-7216-3360-2.
- A academy level textbook focusing on cell biology. Contains information on nucleus structure and function, including nuclear ship, and subnuclear domains
External links
- "The Nucleus". MBInfo.
- "Larn about the Jail cell Nucleus". cellnucleus.com. Website covering construction and office of the nucleus from the Department of Oncology at the Academy of Alberta.
- Bickmore W. "The Nuclear Protein Database". Medical Enquiry Quango Human being Genetics Unit of measurement. Data on nuclear components.
- "The Nucleus Collection". Image & Video Library. The American Society for Prison cell Biological science. Archived from the original on 12 Nov 2006. contains peer-reviewed still images and video clips that illustrate the nucleus.
- Gall JG, McIntosh JR (eds.). "Nuclear Envelope and Nuclear Import Section". Landmark Papers in Cell Biology. Archived from the original on 17 Nov 2006. contains digitized commentaries and links to seminal research papers on the nucleus. Published online in the Epitome & Video Library Archived 10 June 2011 at the Wayback Car of The American Society for Cell Biology
- "Cytoplasmic patterns generated by human antibodies". AntibodyPatterns.com. Archived from the original on 2 Jan 2007.
What Cell Has No Nucleus,
Source: https://en.wikipedia.org/wiki/Cell_nucleus
Posted by: harmonhareand.blogspot.com


0 Response to "What Cell Has No Nucleus"
Post a Comment