
In some respects zinc is chemically similar to magnesium: both elements exhibit merely ane normal oxidation country (+2), and the Zn2+ and Mg2+ ions are of similar size. Corrosion-resistant zinc plating of iron (hot-dip galvanizing) is the major application for zinc. Blanket of steel constitutes the largest unmarried apply of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Nigh 70% of the earth's zinc originates from mining, while the remaining 30% comes from recycling secondary zinc.
Protons and Neutrons in Zinc
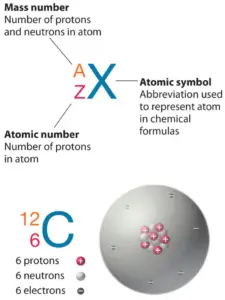 Zinc is a chemical chemical element with diminutive numberxxx which means at that place are 30 protons in its nucleus. Full number of protons in the nucleus is chosen theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals toane,602 ten 10-19 coulombs.
Zinc is a chemical chemical element with diminutive numberxxx which means at that place are 30 protons in its nucleus. Full number of protons in the nucleus is chosen theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals toane,602 ten 10-19 coulombs.
The full number of neutrons in the nucleus of an cantlet is called theneutron number of the atom and is given thesymbol N. Neutron number plus atomic number equals atomic mass number:Due north+Z=A. The difference betwixt the neutron number and the diminutive number is known as theneutron excess: D = N – Z = A – 2Z.
For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same diminutive number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Zinc are64; 66-68; seventy.
Main Isotopes of Zinc
Five stable isotopes of zinc occur in nature, with64Zn beingness the near abundant isotope (49.17% natural affluence).
Zinc-64 is composed of 30 protons, 34 neutrons, and thirty electrons.
Zinc-66 is composed of 30 protons, 36 neutrons, and 30 electrons.
Zinc-67 is composed of 30 protons, 37 neutrons, and 30 electrons.
Zinc-68 is composed of xxx protons, 38 neutrons, and thirty electrons.
Zinc-70 is composed of 30 protons, 40 neutrons, and thirty electrons.
Stable Isotopes
| Isotope | Abundance | Neutron Number |
| 64Zn | 49.two% | 34 |
| 66Zn | 27.7% | 36 |
| 67Zn | 4% | 37 |
| 68Zn | 18.5% | 38 |
| 70Zn | 0.6% | 40 |
Electrons and Electron Configuration
The number of electrons in an electrically-neutral cantlet is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Zinc is 30. Each electron is influenced by the electrical fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the cantlet.
Since the number of electrons and their arrangement are responsible for the chemic behavior of atoms, theatomic number identifies the various chemical elements. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element's electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in guild of increasing atomic number Z.
Electron configuration ofZincis[Ar] 3d10 4s2.
Possible oxidation states are+2.
Zinc has an electron configuration of [Ar]3d104s2 and is a member of the grouping 12 of the periodic tabular array. Information technology is a moderately reactive metal and strong reducing agent. The surface of the pure metal tarnishes speedily, eventually forming a protective passivating layer of the bones zinc carbonate,Zn5(OH)6(CO3)2 , by reaction with atmospheric carbon dioxide.
The chemistry of zinc is dominated by the +two oxidation country. When compounds in this oxidation state are formed, the outer vanquishs electrons are lost, yielding a bare zinc ion with the electronic configuration [Ar]3d10.
Near Important Alloy of Zinc
Zamak is a family of alloys with a base metallic of zinc and alloying elements of aluminium, magnesium, and copper. Alloys of zinc with minor amounts of copper, aluminium, and magnesium are useful in dice casting equally well as spin casting, especially in the automotive, electrical, and hardware industries. Zinc alloys have low melting points, require relatively depression rut input, exercise not require fluxing or protective atmospheres. Because of their high fluidity, zinc alloys can exist cast in much thinner walls than other die castings alloys, and they can exist die bandage to tighter dimensional tolerances.
About Protons
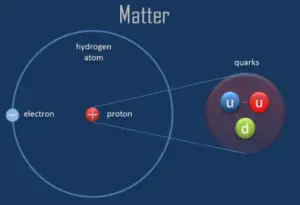 A proton is one of the subatomic particles that make up matter. In the universe, protons are arable, making upabout half of all visible thing. It hasa positive electric charge (+1e) and a balance mass equal to 1.67262 × 10−27 kg (938.272 MeV/c 2 )— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a hateful foursquare radius of about 0.87 × ten−15 m, or 0.87 fm, and it is a spin – ½ fermion.
A proton is one of the subatomic particles that make up matter. In the universe, protons are arable, making upabout half of all visible thing. It hasa positive electric charge (+1e) and a balance mass equal to 1.67262 × 10−27 kg (938.272 MeV/c 2 )— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a hateful foursquare radius of about 0.87 × ten−15 m, or 0.87 fm, and it is a spin – ½ fermion.
The protons be in the nuclei of typical atoms, along with their neutral counterparts, the neutrons. Neutrons and protons, commonly chosennucleons, are spring together in the atomic nucleus, where they account for 99.9 pct of the atom'south mass. Research in high-energy particle physics in the 20th century revealed that neither the neutron nor the protonis not the smallest building block of thing.
About Neutrons
A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are arable, making upmore than half of all visible matter. Information technology hasno electric charge and a balance mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton only nearly 1839 times greater than that of the electron. The neutron has a hateful square radius of most 0.8×10−15 1000, or 0.8 fm, and information technology is a spin-½ fermion.
Diminutive nuclei consist of protons and neutrons, which attract each other throughthe nuclear force, while protons repel each other viathe electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. In that location are only certain combinations of neutrons and protons, which formsstable nuclei.
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electric repulsion between protons. Equally a result, as the number of protons increases,an increasing ratio of neutrons to protons is needed to form a stable nucleus. If in that location are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay.Unstable isotopesdisuse through various radioactive decay pathways, near normally blastoff decay, beta decay, or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known. It should be noted that all of these decay pathways may be accompanied bythe subsequent emission of gamma radiation. Pure alpha or beta decays are very rare.
Well-nigh Electrons and Electron Configuration
The periodic table is a tabular display of the chemic elements organized on the ground of their atomic numbers, electron configurations, and chemic properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of theelectron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Thechemical backdrop of the cantlet are determined by the number of protons, in fact, by number andarrangement of electrons. Theconfiguration of these electrons follows from the principles of breakthrough mechanics. The number of electrons in each element's electron shells, especially the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
It is thePauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the footing state. The ordering of the electrons in the ground state of multielectron atoms, starts with the everyman energy state (ground country) and moves progressively from there up the energy scale until each of the atom's electrons has been assigned a unique set up of quantum numbers. This fact has fundamental implications for the building up of the periodic tabular array of elements.
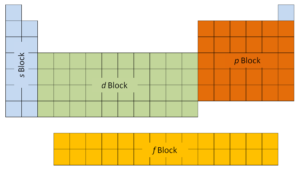 The offset two columns on the left side of the periodic table are where thes subshells are being occupied. Considering of this, the first two rows of the periodic table are labeled thes block. Similarly, thep blockare the right-almost six columns of the periodic table, thed blockis the middle x columns of the periodic table, while thef blockis the 14-cavalcade section that is usually depicted as discrete from the main body of the periodic table. It could be part of the main body, just then the periodic tabular array would be rather long and cumbersome.
The offset two columns on the left side of the periodic table are where thes subshells are being occupied. Considering of this, the first two rows of the periodic table are labeled thes block. Similarly, thep blockare the right-almost six columns of the periodic table, thed blockis the middle x columns of the periodic table, while thef blockis the 14-cavalcade section that is usually depicted as discrete from the main body of the periodic table. It could be part of the main body, just then the periodic tabular array would be rather long and cumbersome.
For atoms with many electrons, this note tin become lengthy and and so an abbreviated note is used. The electron configuration tin be visualized as the core electrons, equivalent to thenoble gas of the preceding period, and the valence electrons (e.k. [Xe] 6s2 for barium).
Oxidation States
Oxidation states are typically represented by integers which may exist positive, nada, or negative. Virtually elements have more than one possible oxidation state. For example, carbon has 9 possible integer oxidation states from −iv to +iv.
The electric current IUPAC Gold Book definition of oxidation state is:
"Oxidation state of an atom is the charge of this cantlet afterward ionic approximation of its heteronuclear bonds…"
and the term oxidation number is nearly synonymous. An chemical element that is non combined with any other different elements has an oxidation country of 0. Oxidation country 0 occurs for all elements – it is simply the element in its elemental course. An atom of an element in a compound volition have a positive oxidation state if information technology has had electrons removed. Similarly, adding electrons results in a negative oxidation state. We have too distinguish betwixt the possible and common oxidation states of every element. For example, silicon has nine possible integer oxidation states from −4 to +4, but only -4, 0 and +4 are mutual oxidation states.
Summary
| Element | Zinc |
| Number of protons | 30 |
| Number of neutrons (typical isotopes) | 64; 66-68; seventy |
| Number of electrons | 30 |
| Electron configuration | [Ar] 3d10 4s2 |
| Oxidation states | +ii |

Source: www.luciteria.com
Other properties of Zinc

 Zinc is a chemical chemical element with diminutive numberxxx which means at that place are 30 protons in its nucleus. Full number of protons in the nucleus is chosen theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals toane,602 ten 10-19 coulombs.
Zinc is a chemical chemical element with diminutive numberxxx which means at that place are 30 protons in its nucleus. Full number of protons in the nucleus is chosen theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals toane,602 ten 10-19 coulombs.

 A proton is one of the subatomic particles that make up matter. In the universe, protons are arable, making upabout half of all visible thing. It hasa positive electric charge (+1e) and a balance mass equal to 1.67262 × 10−27 kg (938.272 MeV/c 2 )— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a hateful foursquare radius of about 0.87 × ten−15 m, or 0.87 fm, and it is a spin – ½ fermion.
A proton is one of the subatomic particles that make up matter. In the universe, protons are arable, making upabout half of all visible thing. It hasa positive electric charge (+1e) and a balance mass equal to 1.67262 × 10−27 kg (938.272 MeV/c 2 )— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. The proton has a hateful foursquare radius of about 0.87 × ten−15 m, or 0.87 fm, and it is a spin – ½ fermion. The offset two columns on the left side of the periodic table are where thes subshells are being occupied. Considering of this, the first two rows of the periodic table are labeled thes block. Similarly, thep blockare the right-almost six columns of the periodic table, thed blockis the middle x columns of the periodic table, while thef blockis the 14-cavalcade section that is usually depicted as discrete from the main body of the periodic table. It could be part of the main body, just then the periodic tabular array would be rather long and cumbersome.
The offset two columns on the left side of the periodic table are where thes subshells are being occupied. Considering of this, the first two rows of the periodic table are labeled thes block. Similarly, thep blockare the right-almost six columns of the periodic table, thed blockis the middle x columns of the periodic table, while thef blockis the 14-cavalcade section that is usually depicted as discrete from the main body of the periodic table. It could be part of the main body, just then the periodic tabular array would be rather long and cumbersome.
0 Response to "Number Of Electrons In Zn"
Post a Comment